Excipients are the unsung heroes of the oral solid-dosage-form (OSDF) pharmaceutical industry, seemingly content to remain out of the limelight and let the active ingredients take center stage. Now, a new breed of specially formulated blends could be just what the doctor ordered to help drug makers optimize production and potentially reduce the cost of producing life-saving medications.
Most consumers are completely unaware of the critical role excipients play in delivering the beneficial medicines millions rely on to fight diseases and stay healthy. Yet it is the fillers, binders, disintegrants, lubricants, and coatings that ensure the accurate, timed delivery of drugs required to safely maintain optimum active ingredient levels in our system and give the OSDF drugs we take every day their palpable size, texture, and flavor.
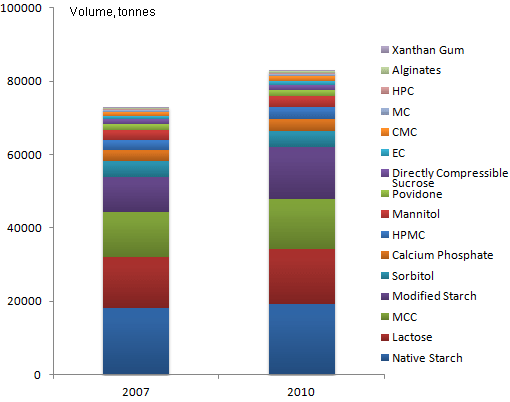
With average growth forecast to exceed 4% through 2015, the excipients market for OSDF is expected to keep pace with the 6% growth on the pharmaceutical horizon. While the two industries commonly track together for obvious reasons, the volume-based excipients market typically lags the value-based drug market.
Challenges at Home and Abroad
U.S. excipients manufacturers are facing some formidable new challenges. Consolidation in the pharmaceuticals industry is beginning to shrink the number of potential customers as merged/acquired drug makers tap into pre-existing supplier agreements with their new partners. Increased focus by the U.S. Food and Drug Administration on the quality and documentation of OSDF clients is resulting in tighter regulation and oversight.
Meanwhile, growth in the outsourcing of drug manufacturing to lower-production-cost countries in Southeast Asia and South America is whittling away at domestic market share. And, in the fillers/binders business, a run-up in the price of pulp, particularly carboxymethylcellulose, is driving up the cost of cellulosics and contributing to the shift of manufacturing/tableting operations to developing markets like India and China.
Stable Market Drives Steady Growth
Despite these challenges, the competitive landscape and overall market remains quite stable. For the most part, there is stability at the customer base, as drug makers are prone to sticking with a formulation that works, rather than make changes and risk alterations in the safe delivery, timed release or effectiveness of their product. Foreign competition from imported excipients remains a minor concern, as most manufacturers are hesitant to switch to more cheaply made Asian ingredients over fears of specification adherence, as well as the real or imagined risk of reduced quality or issues such as radiation contamination.
With the market for generic drugs still going strong, thanks in large part to the recession and continued weak economy, this tried-and-true formulation business has enabled excipient manufacturers to worry more about maintaining market share and less about technological innovation. However, as the economic cloud lifts, excipient manufacturers are shifting their attention back to R&D, working to develop new formulas to optimize drug delivery for the patient and manufacturing processes for drug makers.
Innovative Coatings, Disintegrants Support New Drug Systems
One of the most quickly evolving areas is the formulated coatings business, where interest is growing in both instant- and controlled-release products. Instant-release coatings enhance the drug flavor, making them more palatable, and enable the rapid delivery of medication to speed relief of pain and other symptoms. On the other end of the spectrum, it turns out some patients are not very compliant about taking their maintenance drugs when they should. Controlled release products that combat this problem and deliver sustained effects are also growing at about 5.5%.
Another fascinating area of growth and innovation is the disintegrants market, which is expected to add 5% over the next four years. By enhancing the rate at which the drug is made available to the body, these agents play a critical role in disintegration of the tablet as well as dissolution of the active ingredient. For the patient, it is the disintegrant that enables tablets and capsules to pack a powerful punch of medication in a tiny form that is easier to swallow, particularly for children and the elderly. The demand for rapid-relief, fast-melt tablets is driving 6% volume growth in crospovidone, which has become the disintegrant of choice for its better solubility and functionality compared to sodium starch glycolate and croscamellose sodium.
Blends: The Next Big Thing
Without a doubt, the biggest opportunity in the oral solid-dosage-form is in the development of custom-blended products that provide a one-stop-shop supply for drug makers. Pharmaceutical makers are eager to optimize their processes and reduce production. Customized blends or packaging programs that include a binder, filler, disintegrant, and coating specifically designed for the clients’ drug delivery goals allow the drug manufacturer to have greater control over their active ingredient, and save time and money in the blending process.
The problem is most excipient suppliers are not set up for this kind of broader service and customer-intimate relationship. In fact, the majority is very product-centric, and the industry as a whole is quite siloed in terms of expertise. However, this environment is actually quite perfect for strategic partnerships and alliances with non-competing suppliers of complementary products to meet drug makers’ more specific needs.
In fact, we’ve already seen early signs of this trend with the 2007 “controlled release alliance” between Dow and Colorcon. The deal, aimed at providing a broader solution set and more agile product development to drug makers, combines Dow’s expertise in controlled-release cellulosics with Colorcon’s proven experience and deep portfolio in the formulated coatings business.
Calling the alliance, “a natural progression for both companies in providing increased value to the pharmaceutical formulator,” Colorcon VP Jim Coward also accurately characterized the opportunity in custom blends for the entire industry. It is a natural progression that will no doubt better meet the needs of the market and provide for exciting innovations that will drive growth for years to come.
Anna Ibbotson, Industry Manager